

岭南现代临床外科 ›› 2020, Vol. 20 ›› Issue (02): 238-242.DOI: 10.3969/j.issn.1009-976X.2020.02.022
摘要:
慢性阻塞性肺疾病急性加重期(acute exacerbations of chronic obstructive pulmonary disorder,AECOPD)的死亡风险很高,常需要重症监护室(ICU)治疗,反复发作使患者可能需要长时间住院[1,2]。据文献报告 AECOPD 的比例为每年 100~150例/百万,占有ICU病床的比例约为2%,20%的AECOPD患者需要机械通气[3]。目前对AECOPD患者的流行病学和生理学特征及预后了解有限[4],遗传性相关基因与环境因素相互作用与疾病的发生与发展相关[5]。CYP2A6基因与COPD的病理生理机制有关联,这种关联是建立在COPD与吸烟者关系的基础上[6],即CYP2A6对尼古丁及烟碱的代谢发挥主要作用,CYP2A6基因多态性可能影响COPD患者机体氧化代谢,CYP2A6基因多态性与COPD的密切关系可能对AECOPD产生影响[7]。本课题通过检测2016年4月~2019年4月在我院治疗的AECOPD患者CYP2A6基因型(野生型组、缺失型组),分析与比较两组间患者的临床特征。
本研究采用前瞻性队列研究设计。研究对象为2016年4月~2019年4月在我院接受治疗的AECOPD患者,通过检测患者血浆CYP2A6基因型(分别被指定为野生型、缺失型或突变型)将患者分组为CYP2A6野生型组和CYP2A6缺失型。入组标准:①年龄>50岁,符合2016年COPD全球倡议急性加重诊断标准的患者[8];②排除患有巨大肺大泡、职业性肺纤维化、弥漫性支气管扩张、支气管哮喘和肺癌患者。最终,133例符合入选标准患者入组,患者填写知情同意书,并将课题研究报告一起报医院伦理委员会批准。基线特征见表1。
入院时记录患者基本病史(包括吸烟史、合并症、既往住院治疗数据等)、住院时的生理生化参数和血气分析数据。还包括本次ICU和住院时间、有创通气状况ICU和住院死亡率(表1)。
采用限制性片段长度多态性聚合酶链反应进行检测。取患者住院留取的枸橼酸钠抗凝血2 mL,3000 rmp离心1 min,弃上清,加裂解液,56℃水浴30 min,沸水煮10 min,流水冷却约50 s,震荡器再次剧烈震荡约100 s;最后11 500 rpm高速离心4 min,上清液中即含有模板DNA。CYP2A6基因分型:首先PCR扩增,根据文献[9]由上海生工生物工程有限公司合成CYP2A6引物Kd1F(5′-CCTGATCGACTAGGCGTGGTA-3′)和 E3R(5′-TCGTCCTGGGTGTTTTCCTTC-3′),目的片断大小为215 bp。PCR反应体系为25 μL,其中含DNA模板100~200 ng,上下游引物各10 pmol,4× dNTPs 20 pmol,TaqDNA 聚合酶0.5 U,10×buffer 2.5 μL。反应条件为:预变性94℃2 min,变性94℃ 30 s,退火56℃ 45s,延伸72℃ 1 min,35个循环,最后终止延伸72℃5 min。最后通过限制性片断长度多态性(RFLP)分析已扩增出的CYP2A6基因产物,使用MspI、XcmI、DdeI内切酶对PCR产物进行酶切,鉴定基因型。方法为取PCR扩增产物12 μL,加内切酶5 IU,酶切buffer 2 μL,再加双蒸水至总体积20 μL,于37℃温育4 h,然后进行3%琼脂糖凝胶电泳。
所有观察与检测的数据变量以均数±标准差、四分位数、百分比表示,采用SPSS 20.0进行统计分析。组间比较采用t检验、Kruskal-Wallis和卡方检验(或Fisher精确检验)。P值小于0.05被认为具有统计学意义。
共纳入133例患者,包括CYP2A6野生型108例,CYP2A6缺失型25例,总平均年龄为59.6±18.1岁,患者多为男性(56.3%),详细结果见表1。CYP2A6野生型吸烟例数、急诊入院例数多于缺失型患者,两组间差异有统计学意义(分别P<0.001)。频繁加重次数、入院ICU类型、死亡率、住院时间、APECHEⅡ评分、当日机械通气和肾替代治疗例数以及伴随疾病在两组间没有统计学意义上的差异。
表1 症监护室COPD与非COPD患者基线特征及预后比较
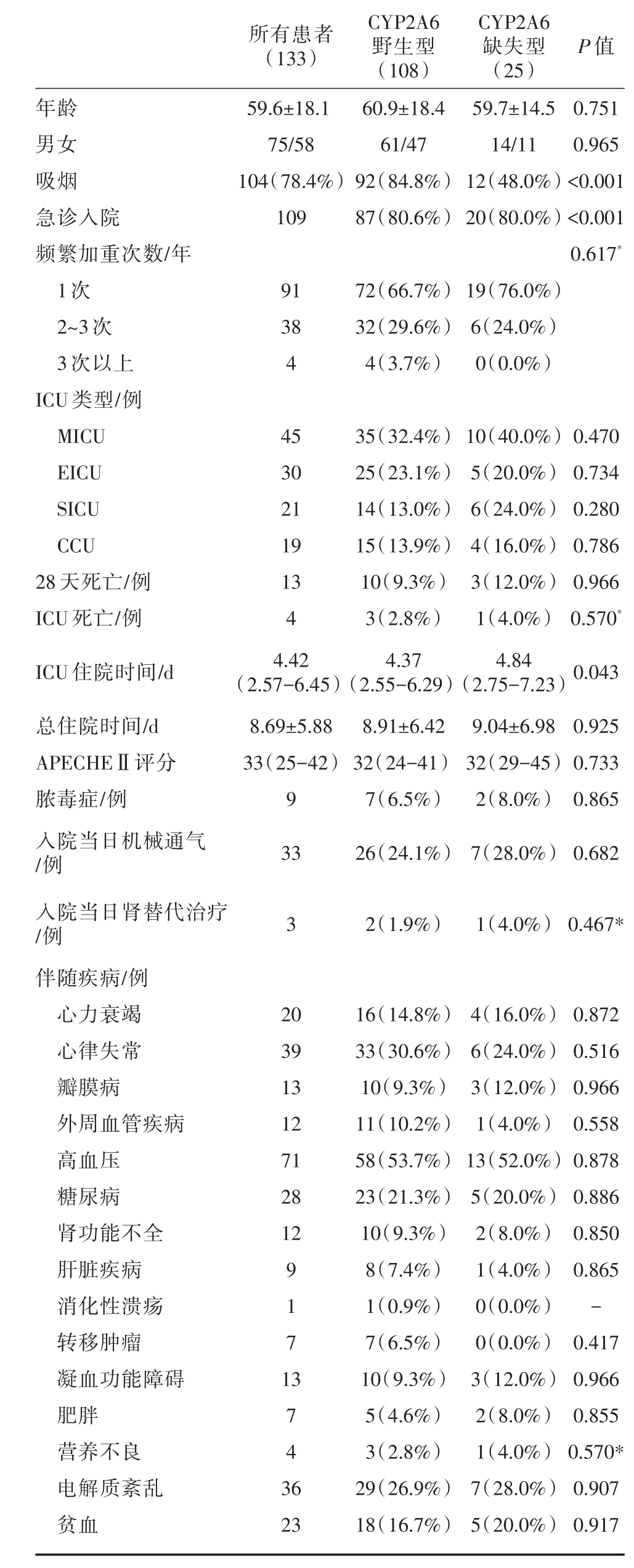
注:数据表示为平均值±标准差、四分位数、例数(%)。COPD,慢性阻塞性肺疾病;MICU,医学重症监护室;CCU,冠状动脉监护室;SICU,外科重症监护室;ICU,重症监护室;APECHEⅡ,急性生理和慢性健康状况评分;*Fishers′精确检验
所有患者(133)P值年龄男女吸烟急诊入院频繁加重次数/年1次2~3次3次以上ICU类型/例MICU EICU SICU CCU 28天死亡/例ICU死亡/例ICU住院时间/d总住院时间/d APECHEⅡ评分脓毒症/例入院当日机械通气/例入院当日肾替代治疗/例伴随疾病/例心力衰竭心律失常瓣膜病外周血管疾病高血压糖尿病肾功能不全肝脏疾病消化性溃疡转移肿瘤凝血功能障碍肥胖营养不良电解质紊乱贫血59.6±18.1 75/58 104(78.4%)109 CYP2A6野生型(108)60.9±18.4 61/47 92(84.8%)87(80.6%)CYP2A6缺失型(25)59.7±14.5 14/11 12(48.0%)20(80.0%)0.751 0.965<0.001<0.001 0.617*91 38 4 72(66.7%)32(29.6%)4(3.7%)19(76.0%)6(24.0%)0(0.0%)45 30 21 19 13 4 4.42(2.57-6.45)8.69±5.88 33(25-42)9 33 35(32.4%)25(23.1%)14(13.0%)15(13.9%)10(9.3%)3(2.8%)4.37(2.55-6.29)8.91±6.42 32(24-41)7(6.5%)26(24.1%)10(40.0%)5(20.0%)6(24.0%)4(16.0%)3(12.0%)1(4.0%)4.84(2.75-7.23)9.04±6.98 32(29-45)2(8.0%)7(28.0%)0.470 0.734 0.280 0.786 0.966 0.570*0.043 0.925 0.733 0.865 0.682 32(1.9%)1(4.0%)0.467*20 39 13 12 71 28 12 9 1 7 1 3 7 4 3 6 23 16(14.8%)33(30.6%)10(9.3%)11(10.2%)58(53.7%)23(21.3%)10(9.3%)8(7.4%)1(0.9%)7(6.5%)10(9.3%)5(4.6%)3(2.8%)29(26.9%)18(16.7%)4(16.0%)6(24.0%)3(12.0%)1(4.0%)13(52.0%)5(20.0%)2(8.0%)1(4.0%)0(0.0%)0(0.0%)3(12.0%)2(8.0%)1(4.0%)7(28.0%)5(20.0%)0.872 0.516 0.966 0.558 0.878 0.886 0.850 0.865-0.417 0.966 0.855 0.570*0.907 0.917
CYP2A6野生型组与CYP2A6缺失型组中分别有24.1%(20/108)和28%(7/25)的患者入院第一天PaO2、PaCO2、血pH在正常范围内;高血糖、高肌酐血症在在正常范围分别为33例(33/108,22.1%)和12.0%(3/25,12.0%);两组白细胞计数均升高;FEV1%预计值分别为47.7±12.3和49.8±12.1;使用噻托溴铵的分别为34.1%(35/108)和28.1%(7/25)。两组生化及生理指标的比较见表2。
从表2中显示,血气分析结果在CYP2A6野生型组波动幅度较大,CYP2A6野生型患者具有更低PaO2、更高的 PaCO2和 HCO3-、高肌酐血症,心率变化幅度较大,与CYP2A6缺失型比较,差异有统计学意义(所有P<0.05)。
频繁发生急性加重的COPD患者在临床上常见,一般认为,每年急性加重发作≥2次,并有1次需要住院,两次间隔时间4 w以上,未治疗的患者应与前一次急性加重始发时间间隔≥6 w,可判定为频繁急性加重型[10]。AECOPD频繁发生的影响因素较复杂,主要包括基础炎症、气道微生物模式、遗传易感性等[11]。
AECOPD的基因多态性是频繁加重型COPD易感性的遗传特点,但目前在机制方面的研究并不完全清楚[12],其中CYP2A6基因多态性与COPD急性加重频率之间关系不断被阐述[13,14]。频繁急性加重COPD患者血浆C-反应蛋白(CRP)、白细胞介素-6(IL-6)等炎症指标进行测定分析中显示,频繁急性加重表型COPD组的上述指标高于COPD组,而携带CYP2A6基因型(野生型)COPD患者中,这种差异在反映氧化应激水平的指标中更为明显,提示CYP2A6基因多态性可以影响COPD患者机体氧化代谢,进而对COPD急性加重产生影响可能[15];CYP2A6基因的多态性也涉及到肺癌的发生发展[16]。
CYP2A6对很多内源及外源性化合物的清除起重要作用,目前已证实人体摄入烟碱的70%~80%经由CYP2A6通过C-氧化途径代谢为可替宁,并进一步将可替宁代谢为反-3-羟基可替宁。在人肝微粒体中,CYP2A6 mRNA、蛋白及其活性水平有着大于50倍的差异,该差异主要由基因型决定。目前已鉴定的CYP2A6基因型有45种,以野生型CYP2A6*1A活性为100%,CYP2A6*1B由于其3‘侧翼区域或3’非翻译区发生点突变,酶活性可能有略微的上升。CYP2A6*4型因为编码区基因序列严重缺失,表型为CYP2A6酶活性缺失型(又称为CYP2A6del型)[9]。等位基因CYP2A6*8的表型则与野生型类似。这些等位基因的存在是引起CYP2A6表达水平及酶活性个体差异的主要原因。由于不同种族人群间CYP2A6等位基因分布频率的不同,又使得CYP2A6酶活性存在较大的种族差异[16]。CYP2A6弱代谢和缺失型个体在人群中占有一定的比例,仅CYP2A6缺失型个体在中国人群中的比例已大于10%。CYP2A6野生基因型COPD患者具有更加严重的肺气肿体症,CYP2A6缺失型患者肺气肿发生率及严重程度均明显降低,因此CYP2A6野生基因型是肺气肿表型的易感因素之一。日本有报道指出不吸烟携带CYP2A6生基因型比不吸烟而烟携带CYP2A6野生缺失型人群,发生COPD概率高6.25倍,而吸烟并携带CYP2A6野生基因型比吸烟并携带CYP2A6野生缺失型人群,发生COPD概率高15.35倍,CYP2A6野生基因型与吸烟在COPD发生、发展过程中有交互作用[6]。
表2 入院第一天急性生化及生理指标
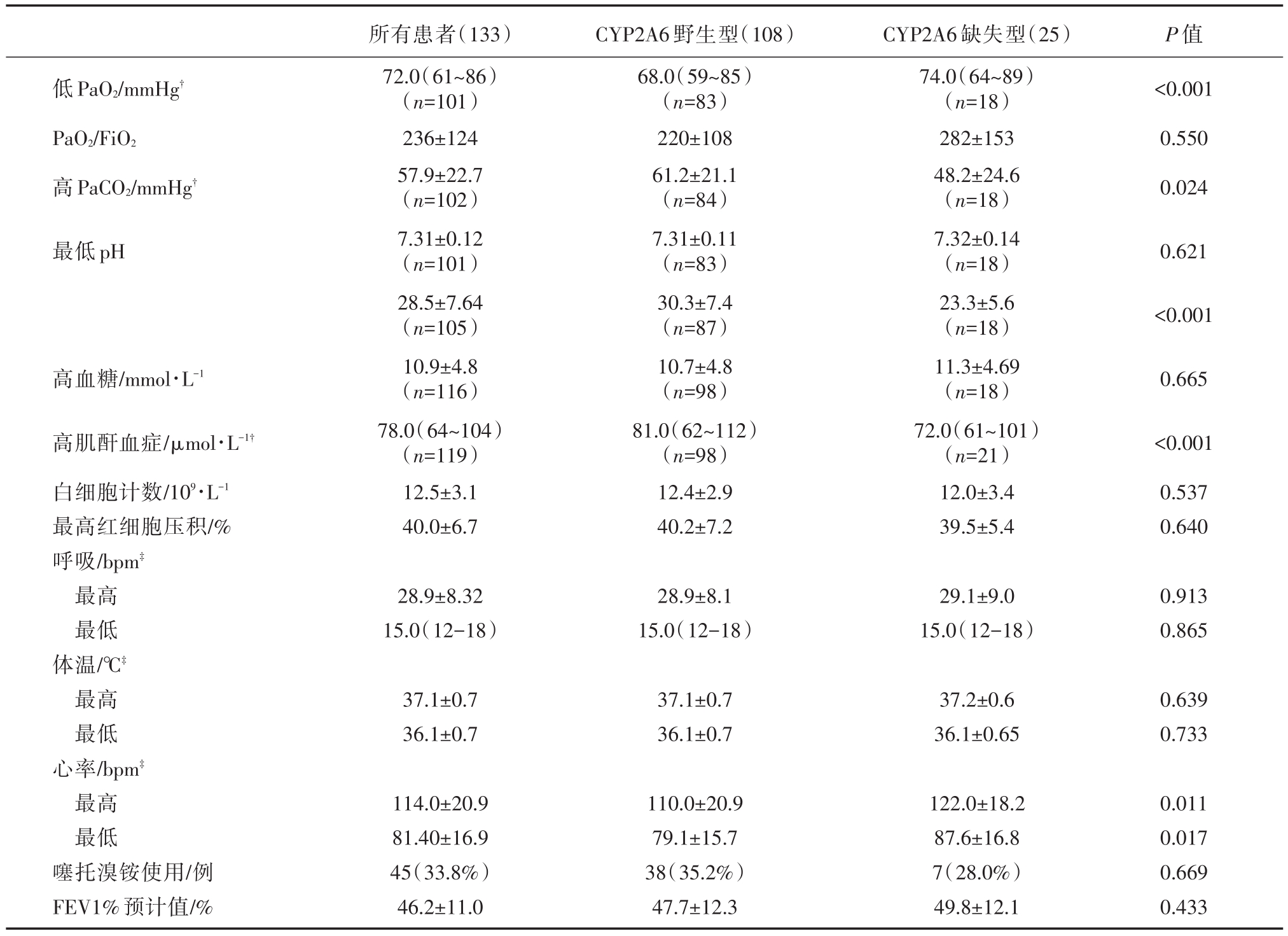
注:?指的是异常PaO2、PaCO2、HCO3-患者的均值,血气分析正常患者不在此列;?指患者在监测过程中出现的最高值和最低值的均值。
低PaO2/mmHg?PaO2/FiO2高PaCO2/mmHg?P值<0.001 0.550 0.024最低pH 0.621<0.001高血糖/mmol·L-10.665高肌酐血症/μmol·L-1?白细胞计数/109·L-1最高红细胞压积/%呼吸/bpm?最高最低体温/℃?最高最低心率/bpm?最高最低噻托溴铵使用/例FEV1%预计值/%所有患者(133)72.0(61~86)(n=101)236±124 57.9±22.7(n=102)7.31±0.12(n=101)28.5±7.64(n=105)10.9±4.8(n=116)78.0(64~104)(n=119)12.5±3.1 40.0±6.7 CYP2A6野生型(108)68.0(59~85)(n=83)220±108 61.2±21.1(n=84)7.31±0.11(n=83)30.3±7.4(n=87)10.7±4.8(n=98)81.0(62~112)(n=98)12.4±2.9 40.2±7.2 CYP2A6缺失型(25)74.0(64~89)(n=18)282±153 48.2±24.6(n=18)7.32±0.14(n=18)23.3±5.6(n=18)11.3±4.69(n=18)72.0(61~101)(n=21)12.0±3.4 39.5±5.4<0.001 0.537 0.640 28.9±8.32 15.0(12-18)28.9±8.1 15.0(12-18)29.1±9.0 15.0(12-18)0.913 0.865 37.1±0.7 36.1±0.7 37.1±0.7 36.1±0.7 37.2±0.6 36.1±0.65 0.639 0.733 114.0±20.9 81.40±16.9 45(33.8%)46.2±11.0 110.0±20.9 79.1±15.7 38(35.2%)47.7±12.3 122.0±18.2 87.6±16.8 7(28.0%)49.8±12.1 0.011 0.017 0.669 0.433
本研究的结果主要显示在CYP2A6野生型吸烟例数、急诊入院例数多于缺失型患者(P<0.001)。血气分析结果反映CYP2A6野生型患者具有更低 PaO2、更高的 PaCO2和 HCO3-,同时CYP2A6野生型患者具有更多高肌酐血症,心率变化幅度较大,与CYP2A6缺失型比较,差异有统计学意义(P<0.05)。以上数据接近文献报道如文献报告CYP2A6野生型严重程度高于缺失型患者,具有更高的吸烟比例等。野生型血肌酐增高比例多可能与疾病严重有关,但无法解释心率变化幅度因素。由于收集资料只限于两组患者的基线资料和入院第一天的生理生化指标,因此本研究的深度不够,同时CYP2A6缺失型患者的例数太少,无法深入进行和数据偏差大,需要更多病例资源和更深入的研究来进一步探讨CYP2A6基因的多态性对于AECOPD疾病的影响。
[1] Mathers CD,Loncar D.Projections of global mortality and burden of disease from 2002 to 2030[J].PLoS Med,2006,3(11):e442.
[2] Seneff MG,Wagner DP,Wagner RP,et al.Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease[J].JAMA,1995,274(23):1852-1857.
[3] Berenyi F,Steinfort DP,Ali Abdelhamid Y,et al.Characteristics and Outcomes of Critically Ill Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Australia and New Zealand[J].Ann Am Thorac Soc,2020.doi:10.1513/AnnalsATS.201911-821OC.[Epub ahead of print]
[4] Molinari N,Chanez P,Roche N,et al.Rising total costs and mortality rates associated with admissions due to COPD exacerbations[J].Respir Res,2016,17(1):149.
[5] Yuan C,Chang,Lu G,Deng X.Genetic polymorphism and chronic obstructive pulmonary disease[J].Int J Chron Obstruct Pulmon Dis,2017,12:1385-1393.
[6] Minematsu N,Nakamura H,Iwata M,et al.Association of CYP2A6 deletion polymorphism with smoking habit and development of pulmonary emphysema[J].Thorax,2003,58(7):623-628.
[7] Keam SJ,Keating GM.Tiotropium bromide.A review of its use as maintenance therapy in patients with COPD [J].Treat Respir Med,2004,3(4):247-268.
[8] Mirza S,Clay RD,Koslow MA,Scanlon PD.COPD Guidelines:A Review of the 2018 GOLD Report[J].Mayo Clin Proc,2018,93(10):1488-1502.
[9] Kitagawa K,Kunugita N,Katoh T,et al.The significance of the homozygous CYP2A6 deletion on nicotine metabolism:a new genotyping method of CYP2A6 using a single PCR-RFLP[J].Biochem Biophys Res Commun,2013,261(1):146-151.
[10] Riley CM,Sciurba FC.Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease:A Review[J].JAMA,2019,321(8):786-797.
[11] Viniol C,Vogelmeier CF.Exacerbations of COPD[J].Eur Respir Rev,2018,27(147).
[12] Qiu W,Cho MH,Riley JH,et al.Genetics of sputum gene expression in chronic obstructive pulmonary disease[J].PLoS One,2011,6(9):e24395.
[13] Gaude GS,Rajesh BP,Chaudhury A,Hattiholi J.Outcomes associated with acute exacerbations of chronic obstructive pulmonary disorder requiring hospitalization[J].Lung India,2015,32(5):465-472.
[14] Ando M,Hamajima N,Ariyoshi N,et al.Association of CYP2A6 gene deletion with cigarette smoking status in Japanese adults[J].J Epidemiol,2003,13(3):176-181.
[15] Davidson MD,Kukla DA,Khetani SR.Microengineered cultures containing human hepatic stellate cells and hepatocytes for drug development[J].Integr Biol(Camb),2017,9(8):662-677.
[16] Zanger UM,Schwab M.Cytochrome P450 enzymes in drug metabolism:regulation of gene expression,enzyme activities,and impact of genetic variation[J].Pharmacol Ther,2013,138(1):103-141.
Characteristics of polymorphism of CPY2A6 gene in patients associated with acute exacerbations of chronic obstructive pulmonary disorder
中图分类号: