

岭南现代临床外科 ›› 2020, Vol. 20 ›› Issue (02): 180-183.DOI: 10.3969/j.issn.1009-976X.2020.02.010
摘要:
早期移植物功能障碍(early allograft dysfunction,EAD)是指肝移植术后由于供肝脂肪变性和缺血再灌注打击下出现供肝功能不全,严重者可出现移植肝无功能,造成受体再移植或死亡。因此了解影响肝移植患者围术期发生EAD的危险因素对优化肝移植诊疗及预后有重要作用。自EAD概念产生以来,对EAD进行定义的肝脏实验室参数一直存在争议,目前,普遍采用Olthoff[1]及其移植团队提出的定义标准:肝移植患者术后第7天国际标准化比值(INR)≥1.6或血清胆红素总量≥10 mg/dL(171 μmol/L),或者术后7 d内谷氨酸转氨酶(ALT)或天冬氨酸转氨酶(AST)>2 000 IU/L,以上条件满足一项即可诊断。本次研究以中山市人民医院2015年1月1日至2019年06月31日进行肝移植的112例患者为研究对象,基于Olthoff定义标准分析EAD发生的危险因素,并探讨供肝的选择,为临床防治提供依据。
选择2015年1月1日至2019年06月31日中山市人民医院进行肝移植患者112例病例,查阅相关资料,包括病历、实验室检查、影像学检查等资料,剔除资料不全者,纳入109例患者资料进行研究。基于Olthoff定义标准,肝移植术后发生EAD患者39例,未发生EAD患者70例,肝移植术后发生EAD患者为EAD组,术后未发生EAD患者(非EAD组)为对照组。
本次纳入的109例肝移植患者均为脑死亡供肝移植,所有供体经过伦理委员会审查通过,所有手术均由胡泽民主任医师作为主刀完成,收集的资料包括受体一般情况和实验室检查(年龄,性别,BMI指数,术前乳酸(LAC)、血清白蛋白、ALT、AST、血清总胆红素、凝血酶原时间、INR、冷缺血时间、热缺血时间、重症监护病房(ICU)住院时间。)、供体一般情况和实验室检查(年龄、性别,体质指数、术前及术后NLR、PAR、血清白蛋白,ALT、AST、血清总胆红素、凝血酶原时间、血小板)、冷缺血时间、手术方式、手术时间、术中出血量等。
采用SPSS软件进行统计分析,符合正态分布计量资料以均数±标准差(x±s)表示,组间比较采用两独立样本t检验,以P<0.05为差异有统计学意义。计数资料组间比较采用χ2检验。多因素分析采用Logistic回归分析,以P<0.05为差异有统计学意义。
EAD组供体术前乳酸(LAC)、供体年龄、受体身体质指数(body mass index,BMI)、受体术前白细胞(WBC)、受体术后淋巴细胞百分比(LYMPH%)、受体术后中性粒细胞/淋巴细胞比值(Neutrophil-to-Lymphocyte Ratio,NLR)、供肝冷缺血时间与非EAD存在差异(P<0.05),见表1。
将以上单因素分析得出的供体年龄,供体BMI、供体术前白蛋白、供体术前中性粒细胞百分比,供体术前LAC,供肝冷缺血时间,受体BMI,受体术前WBC,受体术后WBC、受体术后中性粒细胞百分比、受体术后LYMPH%,受体术后NLR共12个因素(P<0.1),纳入多因素二元Logistic回归分析。结果显示供体术前白蛋白、供体术前LAC、供肝冷缺血时间和受体BMI是肝移植术后发生EAD的独立相关因素(P<0.05)。(详见表2)
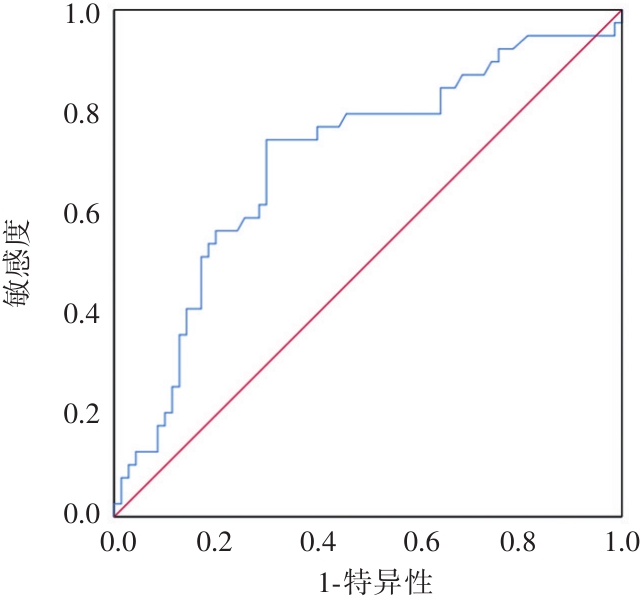
将供体术前白蛋白、供体术前LAC、供肝冷缺血时间和受体BMI分别对肝移植术后EAD预测作ROC曲线得出:供体术前白蛋白曲线下面积(AUC)为0.607,供体术前LAC的(AUC)为0.694,受体BMI曲线下面积(AUC)为0.657,供肝冷缺血时间的AUC为0.703,供肝冷缺血时间ROC曲线下面积均在0.7~0.9之间,提示对术后发生EAD预测具有一定准确度,进一步利用约登指数分析得出:供肝冷缺血时间最佳临界值为313.50 min,利用该切点预测术后发生EAD的灵敏度为74.40%,特异度为70.00%。
冷缺血时间是肝移植术后EAD发生的独立危险因素,这已经成为了普遍的共识[1-3]。本次研究中也发现冷缺血时间在两组间有明显的差异,进一步的研究也提示冷缺血时间≥313.50 min(灵敏度为74.40%,特异度为70.00%)是独立的危险因素,目前文献中各中心得出了不同的截止时间,确定合适的冷缺血截止时间一直是争论的焦点,在肝移植早期普遍认为热缺血时间不应超过5 min,冷缺血时间不应超过8 h,但近些年随着肝移植技术的发展,尤其是机械灌注技术的不断发展,使冷缺血时间被不断延长,有文献报道将冷缺血时间<10 h的供肝进行移植后,移植患者的1年和3年存活率仍能达到81%和67%[4],另一篇来自602例患者的回顾性分析也认为冷缺血时间≥10 h才是肝移植术后EAD的危险因素[5]。本中心得出的冷缺血截止时间符合较早期的结论,这可能是静态低温保存(4~6℃)的极限时间,静态低温保存方法简单且成本较低,是目前主要采用的保存方法,虽然低温保存可以显著降低肝细胞的代谢,但是供肝组织缺血状态下无氧糖酵解不可能完全停止。糖酵解的持续进行造成细胞内乳酸堆积及肝细胞内环境紊乱,引起肝细胞、血管内皮细胞和胆管上皮细胞的变性坏死,导致肝功能受损,缺血再灌注损伤加重。虽然灌注技术已发展出低温机械灌注、亚常温机械灌注和常温机械灌注等不同方式方法[6],但由于其操作复杂、成本昂贵,并不能广泛普及使用。
表1 肝移植术后发生EAD的单因素分析
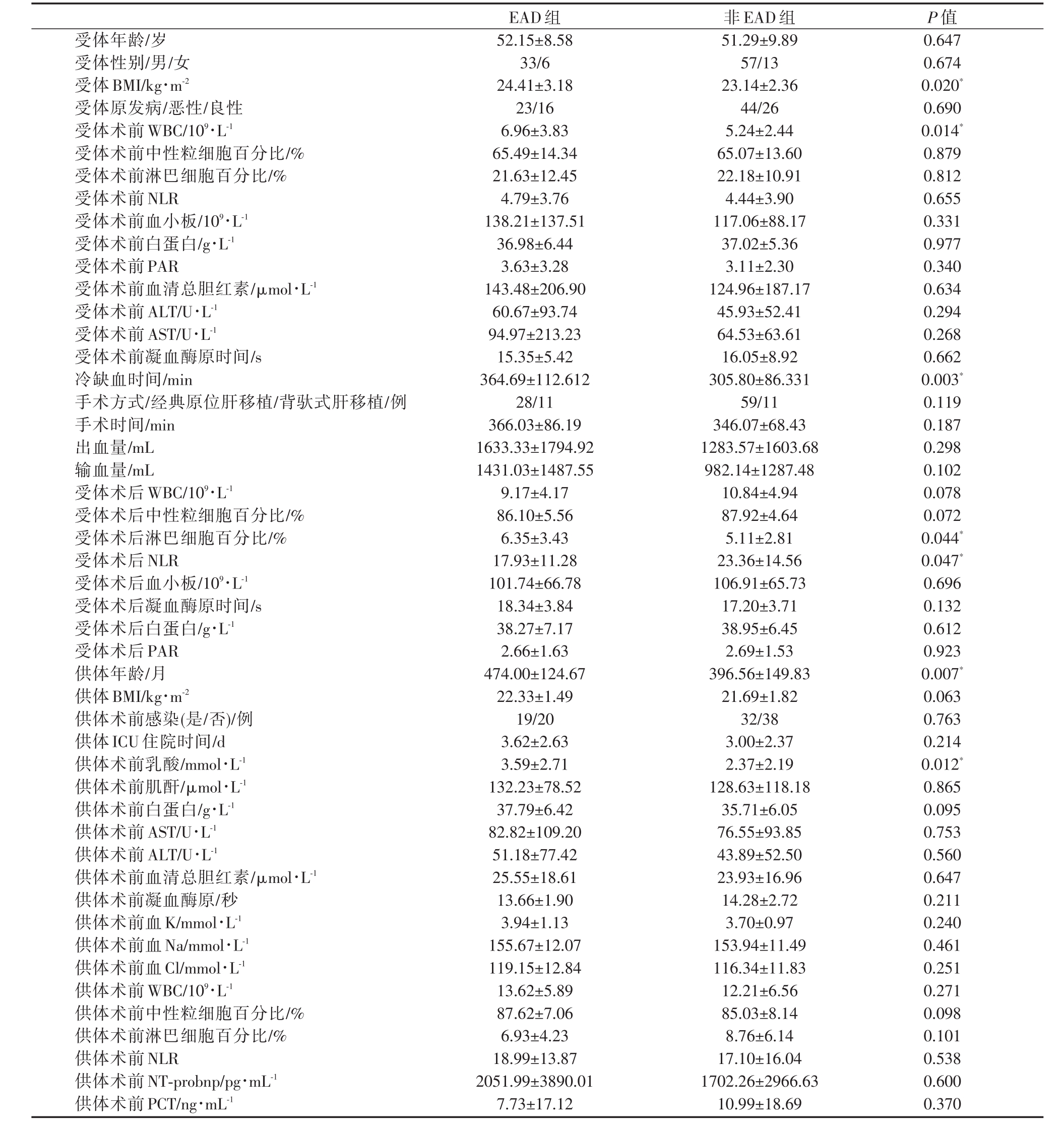
受体年龄/岁受体性别/男/女受体BMI/kg·m-2受体原发病/恶性/良性受体术前 WBC/109·L-1受体术前中性粒细胞百分比/%受体术前淋巴细胞百分比/%受体术前NLR受体术前血小板/109·L-1受体术前白蛋白/g·L-1受体术前PAR受体术前血清总胆红素/μmol·L-1受体术前ALT/U·L-1受体术前AST/U·L-1受体术前凝血酶原时间/s冷缺血时间/min手术方式/经典原位肝移植/背驮式肝移植/例手术时间/min出血量/mL输血量/mL受体术后 WBC/109·L-1受体术后中性粒细胞百分比/%受体术后淋巴细胞百分比/%受体术后NLR受体术后血小板/109·L-1受体术后凝血酶原时间/s受体术后白蛋白/g·L-1受体术后PAR供体年龄/月供体BMI/kg·m-2供体术前感染(是/否)/例供体ICU住院时间/d供体术前乳酸/mmol·L-1供体术前肌酐/μmol·L-1供体术前白蛋白/g·L-1供体术前AST/U·L-1供体术前ALT/U·L-1供体术前血清总胆红素/μmol·L-1供体术前凝血酶原/秒供体术前血K/mmol·L-1供体术前血Na/mmol·L-1供体术前血Cl/mmol·L-1供体术前 WBC/109·L-1供体术前中性粒细胞百分比/%供体术前淋巴细胞百分比/%供体术前NLR供体术前NT-probnp/pg·mL-1供体术前PCT/ng·mL-1 52.15±8.58 33/6 24.41±3.18 23/16 6.96±3.83 65.49±14.34 21.63±12.45 4.79±3.76 138.21±137.51 36.98±6.44 3.63±3.28 143.48±206.90 60.67±93.74 94.97±213.23 15.35±5.42 364.69±112.612 28/11 366.03±86.19 1633.33±1794.92 1431.03±1487.55 9.17±4.17 86.10±5.56 6.35±3.43 17.93±11.28 101.74±66.78 18.34±3.84 38.27±7.17 2.66±1.63 474.00±124.67 22.33±1.49 19/20 3.62±2.63 3.59±2.71 132.23±78.52 37.79±6.42 82.82±109.20 51.18±77.42 25.55±18.61 13.66±1.90 3.94±1.13 155.67±12.07 119.15±12.84 13.62±5.89 87.62±7.06 6.93±4.23 18.99±13.87 2051.99±3890.01 7.73±17.12 51.29±9.89 57/13 23.14±2.36 44/26 5.24±2.44 65.07±13.60 22.18±10.91 4.44±3.90 117.06±88.17 37.02±5.36 3.11±2.30 124.96±187.17 45.93±52.41 64.53±63.61 16.05±8.92 305.80±86.331 59/11 346.07±68.43 1283.57±1603.68 982.14±1287.48 10.84±4.94 87.92±4.64 5.11±2.81 23.36±14.56 106.91±65.73 17.20±3.71 38.95±6.45 2.69±1.53 396.56±149.83 21.69±1.82 32/38 3.00±2.37 2.37±2.19 128.63±118.18 35.71±6.05 76.55±93.85 43.89±52.50 23.93±16.96 14.28±2.72 3.70±0.97 153.94±11.49 116.34±11.83 12.21±6.56 85.03±8.14 8.76±6.14 17.10±16.04 1702.26±2966.63 10.99±18.69 0.647 0.674 0.020*0.690 0.014*0.879 0.812 0.655 0.331 0.977 0.340 0.634 0.294 0.268 0.662 0.003*0.119 0.187 0.298 0.102 0.078 0.072 0.044*0.047*0.696 0.132 0.612 0.923 0.007*0.063 0.763 0.214 0.012*0.865 0.095 0.753 0.560 0.647 0.211 0.240 0.461 0.251 0.271 0.098 0.101 0.538 0.600 0.370
表2 肝移植术后发生EAD多因素Logistic回归分析
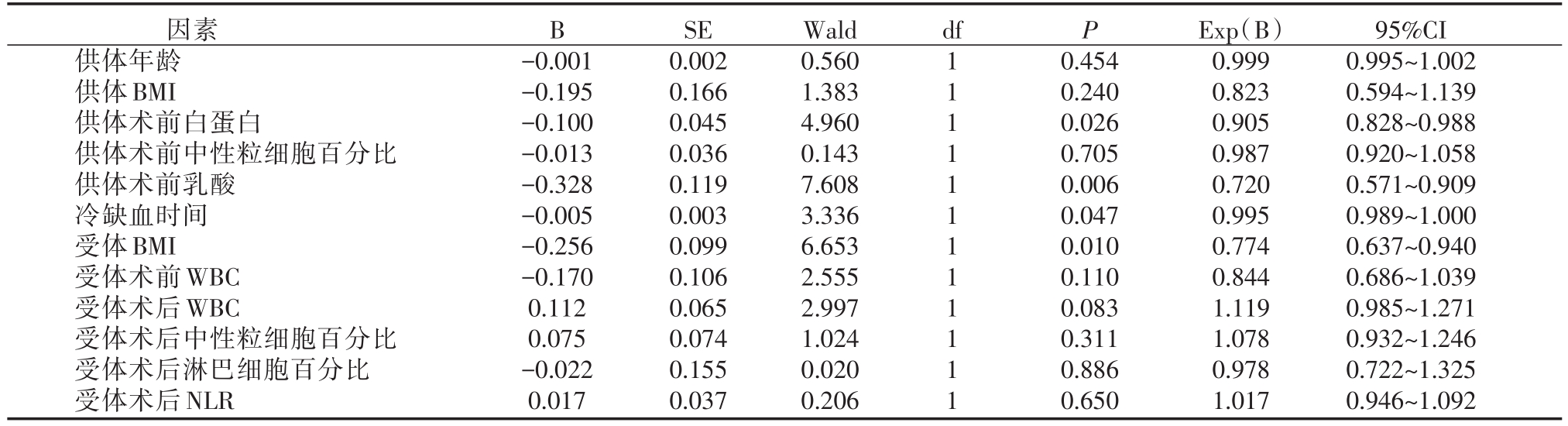
B df P因素供体年龄供体BMI供体术前白蛋白供体术前中性粒细胞百分比供体术前乳酸冷缺血时间受体BMI受体术前WBC受体术后WBC受体术后中性粒细胞百分比受体术后淋巴细胞百分比受体术后NLR-0.001-0.195-0.100-0.013-0.328-0.005-0.256-0.170 0.112 0.075-0.022 0.017 SE 0.002 0.166 0.045 0.036 0.119 0.003 0.099 0.106 0.065 0.074 0.155 0.037 Wald 0.560 1.383 4.960 0.143 7.608 3.336 6.653 2.555 2.997 1.024 0.020 0.206 1 1 1 1 1 1 1 1 1 1 1 1 0.454 0.240 0.026 0.705 0.006 0.047 0.010 0.110 0.083 0.311 0.886 0.650 Exp(B)0.999 0.823 0.905 0.987 0.720 0.995 0.774 0.844 1.119 1.078 0.978 1.017 95%CI 0.995~1.002 0.594~1.139 0.828~0.988 0.920~1.058 0.571~0.909 0.989~1.000 0.637~0.940 0.686~1.039 0.985~1.271 0.932~1.246 0.722~1.325 0.946~1.092
表3 拟合优度检验
-2对数似然Cox and Snell Nagelkerke 97.979a 0.333 0.457
曲线下方区域面积:0.7
本次研究还发现供体相关因素有年龄是独立的危险因素,供体术前LAC是相关危险因素,供体年龄过大不可避免的引起供体器官功能的老化,对缺血再灌注损伤及免疫排斥反应的耐受性较差[7],多篇文献表明供体年龄是EAD的危险因素,但在极限年龄上却存在差异,目前普遍认为供体年龄不应该大于45岁[8,9]。血液LAC是葡糖糖糖酵解的中间产物,其代谢主要在肝脏进行,多篇文献表明肝移植后乳酸清除延迟是EAD的独立危险因素并对肝移植预后有预测能力[10,11]。本中心供体术前LAC值是对供体取肝前2小时内的测定值,在一定程度上反映了供肝的功能状态,供体术前LAC在两组间的差异反映了在肝移植前供肝已经存在不同程度的功能损害,而这种损害可以增加术后EAD的风险。
受体相关因素有受体BMI、受体术前WBC、受体术后LYMPH%、受体术后NLR。目前,受体BMI对EAD的影响尚不清楚,这可能与受者的基础状态有密切关系,一方面,多篇文献表明BMI≥24 kg/m2时与高血脂、糖尿病及高血压呈密切正相关性[12,13],术前高血脂状态可对术后新肝代谢造成沉重负担,引起肝细胞的脂肪变性及坏死。另一方面,超重或肥胖能够提高体内微炎症水平,这可以导致肝移植术后缺血再灌注损伤加重,进而增加EAD的风险[14,15]。此外,本研究还得出围绕肝移植围术期的一些炎症指标在两组间存在明显差异,在相关文献中也报道了不同炎症指标是EAD的危险因素[16-18],炎症介质在缺血再灌注损伤中扮演重要角色,当术后缺血再灌注损伤使肝内微小血管内皮受损时,体内炎症介质即可激活,通过信号传导通路激活炎症反应,而围术期炎症水平增高又可以加速加重此过程。
总之,肝移植冷缺血时间是主要的危险因素,在移植过程中应尽量缩短器官低温保存时间,此外在移植前应尽量调整好受体的一般状态,在围术期的整个过程中减少感染风险,发生感染时及时治疗。
[1] Olthoff KM,Kulik L,Samstein B,et al.Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors[J].Liver Transpl,2010,16(8):943-949.
[2] 周光文.肝移植后原发性移植物功能不良:从发病机制到预防的认识[J].外科理论与实践,2002,7(2):168-170.
[3] Bzeizi KI,Jalan R,Plevris JN,Hayes PC.Primary graft dysfunction after liver transplantation:from pathogenesis to prevention[J].Liver Transpl Surg,1997,3(2):137-48.
[4] Reich DJ,Munoz SJ,Rothstein KD,et al.Controlled non-heartbeating donor liver transplantation:a successful single center experience,with topic update[J].Transplantation,2000,70(8):1159-1166.
[5] Bastos-Neves D,Salvalaggio PRO,Almeida MD.Risk factors,surgical complications and graft survival in liver transplant recipients with early allograft dysfunction[J].Hepatobiliary Pancreat Dis Int,2019,18(5):423-429.
[6] Zhang Y,Zhang Y,Zhang M,et al.Hypothermic machine perfusion reduces the incidences of early allograft dysfunction and biliary complications and improves 1-year graft survival after human liver transplantation:A Meta-analysis[J].Medicine(Baltimore),2019,98(23):e16033.
[7] Johnson S R,Alexopoulos S,Curry M,et al.Primary Nonfunction(PNF)in the MELD Era:An SRTR Database Analysis[J].Am J Transplant,2007,7(4):1003-1009.
[8] Ploeg RJ,D′Alessandro AM,Knechtle SJ,et al.Risk factors for primary dysfunction after liver transplantation--a multivariate analysis[J].Transplantation,1993,55(4):807-813.
[9] Brokelman W,Stel AL,Ploeg RJ.Risk factors for primary dysfunction after liver transplantation in the University of Wisconsin solution era[J].Transplant Proc,1999,31(5):2087-2090.
[10] Takahashi K,Jafri SR,Safwan M,et al.Peri-transplant lactate levels and delayed lactate clearance as predictive factors for poor outcomes after liver transplantation:A propensity scorematched study[J].Clinl Transplant,2019,33(7):e13613.
[11] Kim DG,Lee JY,Jung YB,et al.Clinical significance of lactate clearance for the development of early allograft dysfunction and short-term prognosis in deceased donor liver transplantation[J].Clin Transplantation,2017,31(12):e13136.
[12] 甘小玲,陈庆瑜,廖蓓,等.体重指数对血糖、血脂及血压的影响[J].临床医学,2010,30(6):9-10.
[13] Cong L,Zhan JQ,Yang L,et al.Overweight and obesity among low-income Muslim Uyghur women in far western China:correlations of body mass index with blood lipids and implications in preventive public health[J].PLoS One,2014,9(2):e90262.
[14] Koca TT.Does obesity cause chronic inflammation?The association between complete blood parameters with body mass index and fasting glucose[J].Pak J Med Sci,2017,33(1):65-69.
[15] Ooi GJ,Burton PR,Bayliss J,et al.Effect of Body Mass Index,Metabolic Health and Adipose Tissue Inflammation on the Severity of Non-alcoholic Fatty Liver Disease in Bariatric Surgical Patients:a Prospective Study[J].Obes Surg,2019,29(1):99-108.
[16] Kwon HM,Moon YJ,Jung KW,et al.Neutrophil-to-lymphocyte ratio is a predictor of early graft dysfunction following living donor livertransplantation[J].LiverInt,2019,39(8):1545-1556.
[17] Derbisz K,Nylec M,Chrzaszcz P,et al.Recipient-related preoperative and intraoperative risk factors for primary graft dysfunction after orthotopic liver transplantation[J].Transplant Proc,2018,50(7):2018-2021.
[18] Kwon HM,Jung KW,Moon YJ,et al.Prevalence of antiphospholipid antibody positivity and association of pretransplant lupus anticoagulant positivity with early allograft dysfunction in liver transplantation[J].Transplant Proc,2018,50(4):1136-1141.
Risk factors of early allograft dysfunction after liver transplantation
中图分类号: